Chlorine trifluoride
Chlorine trifluoride is a very toxic and corrosive chemical compound. It is colourless but has a pungent odour. It reacts with water to form hydrochloric acid and oxygen, making it a highly reactive substance.
| Formula | ClF3 |
|---|---|
| Hazards | 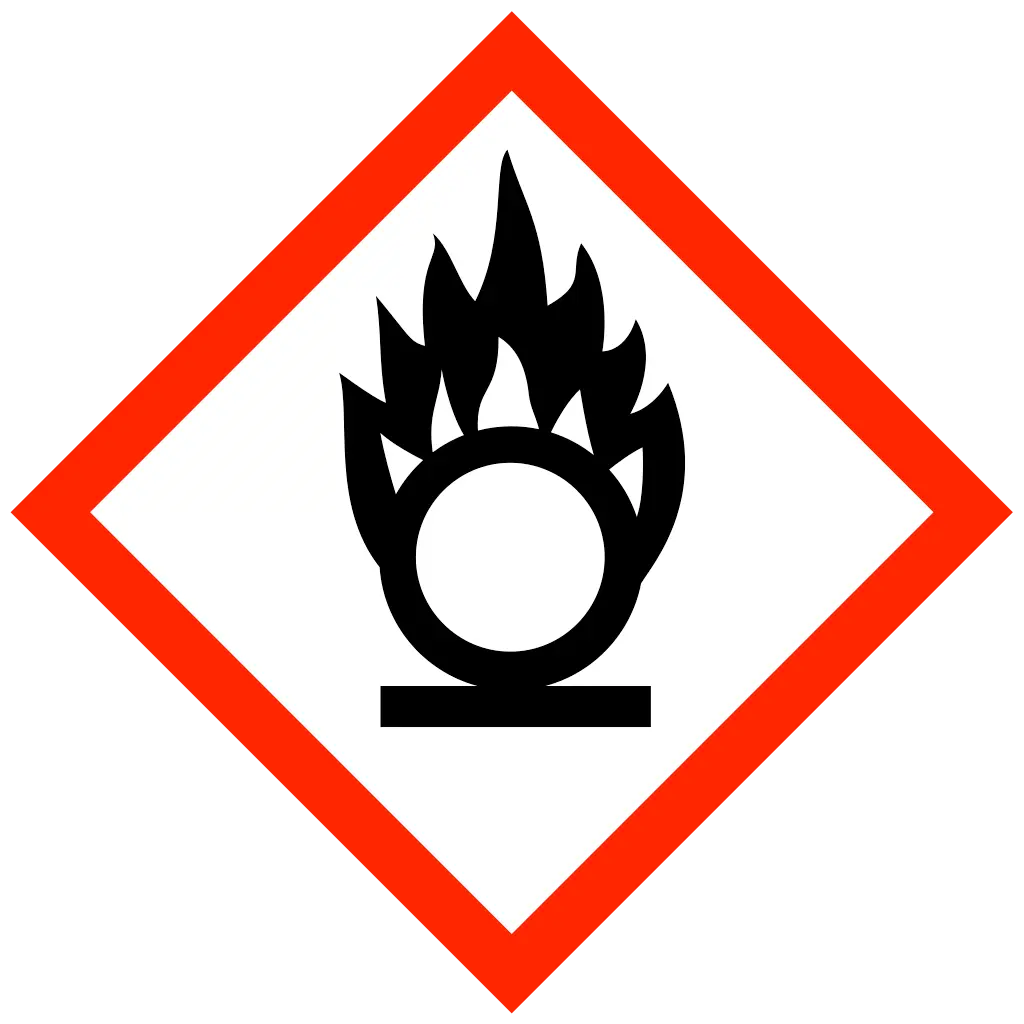 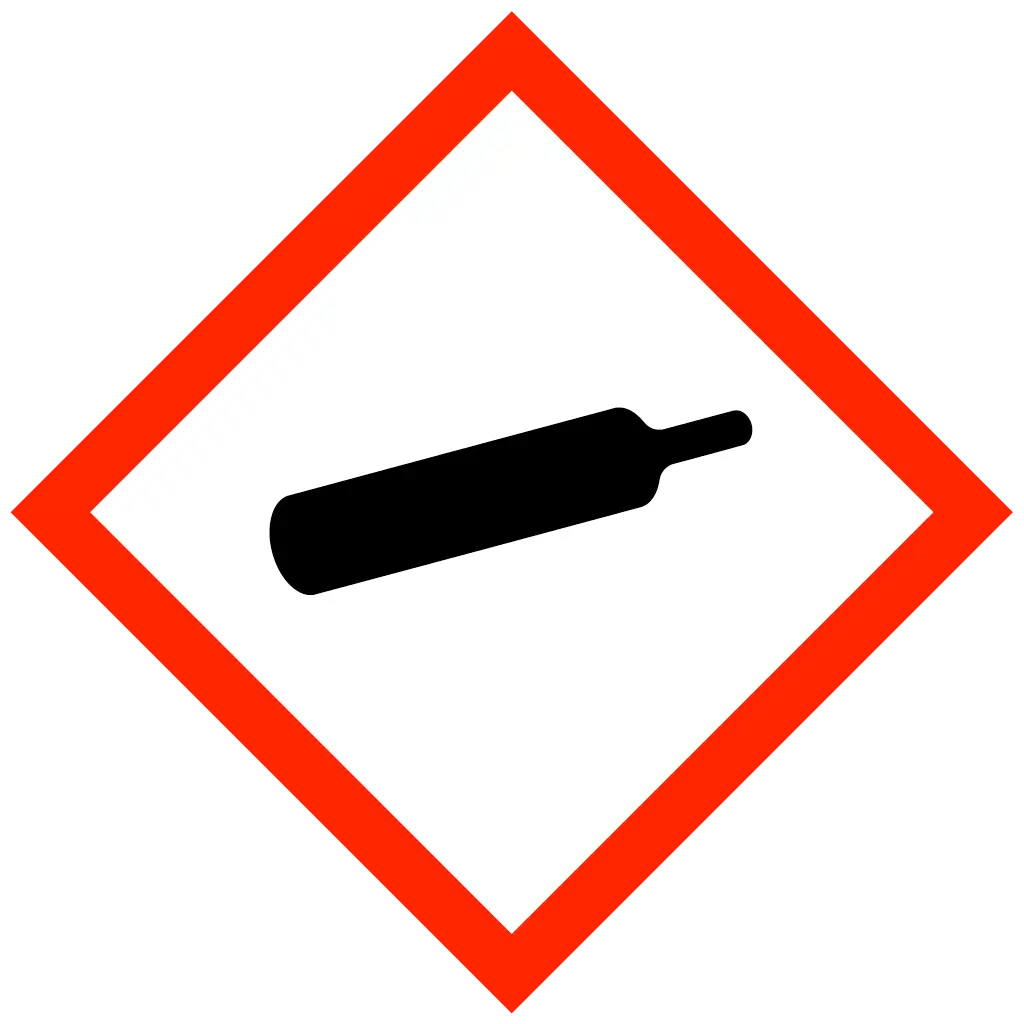 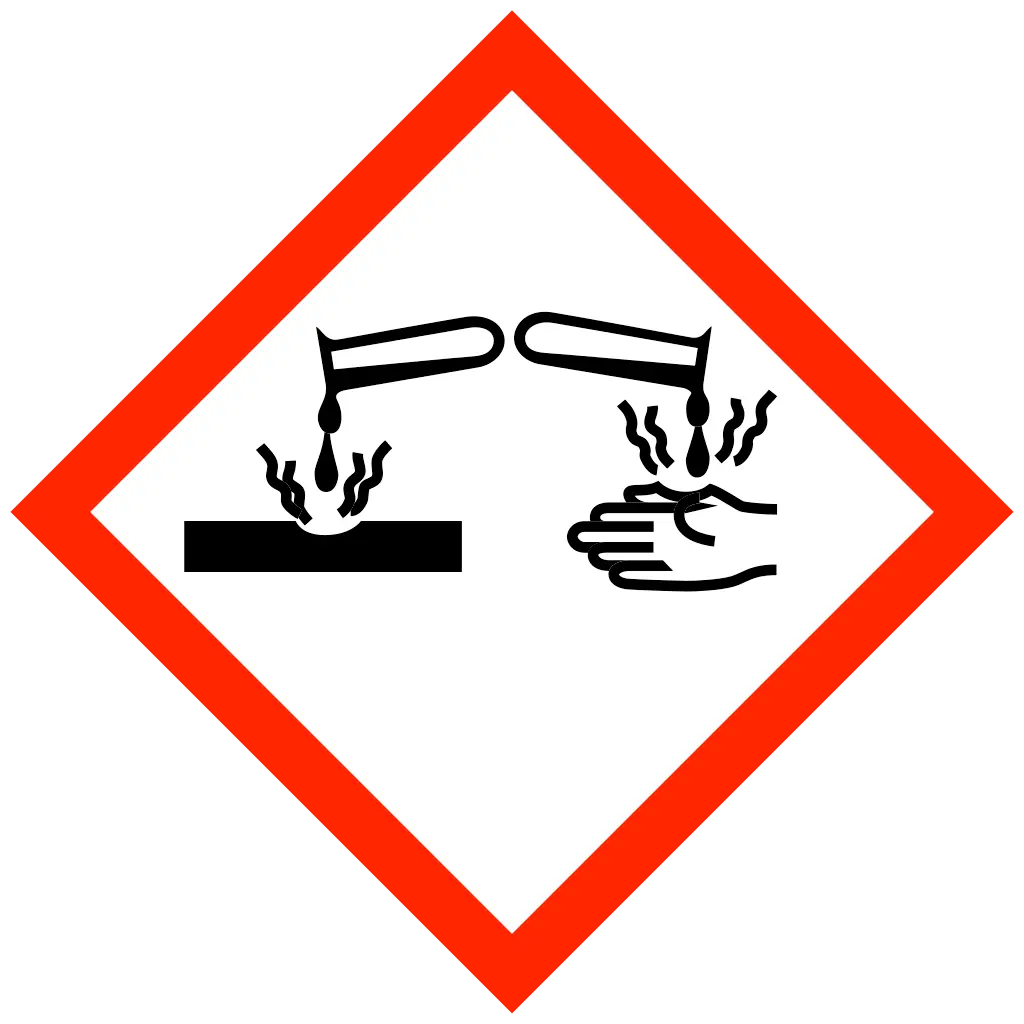  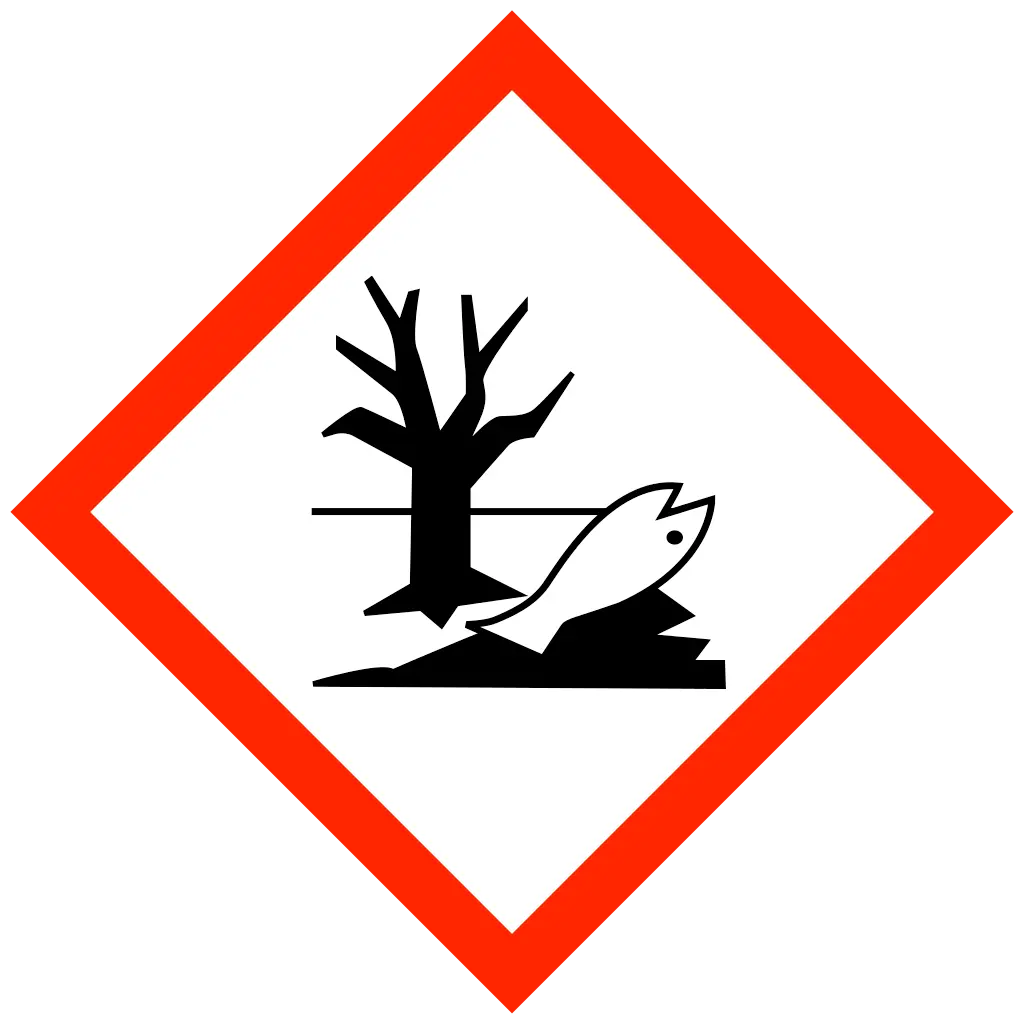 |
| Molecular Weight | 92.45 g/mol |
| Boiling point | 12 °C |
| Melting point | -76 °C |
| Solubility | Reacts with water |
| PubChem CID | 24637 |
Uses
- Fire extinguishing agent
- Rocket propellant
- Used in processing of nuclear fuel to turn Uranium into Uranium hexafluoride
Safety
| Pictograms |      |
|---|---|
| Signal | DANGER |
| Statements | DANGER Oxidising gas DANGER Skin corrosion DANGER Acute toxicity WARNING Gas under pressure WARNING Hazardous to aquatic life |
Fire and Explosion Hazards
Chlorine trifluoride is an oxidiser and can cause or enhance the combustion of other materials. It is highly reactive and can ignite spontaneously in contact with organic materials.
Health Hazards
- Liquid form contact with skin causes third degree burns.
- Severe lung irritation and damage can occur from inhalation.
- Death can occur from inhalation of high concentrations.
References
- Chlorine trifluoride - PubChem
- [Chlorine trifluoride - Wikipedia](https://en.wikipedia.org/wiki/chlorine trifluoride)